
Online Application for Stable Isotope Studies-Groundwater (OASIS-G)
Central Ground Water Board
Department of Water Resources, River Development and Ganga Rejuvenation
Ministry of Jal Shakti
Government of India



Central Ground Water Board
Department of Water Resources, River Development and Ganga Rejuvenation
Ministry of Jal Shakti
Government of India


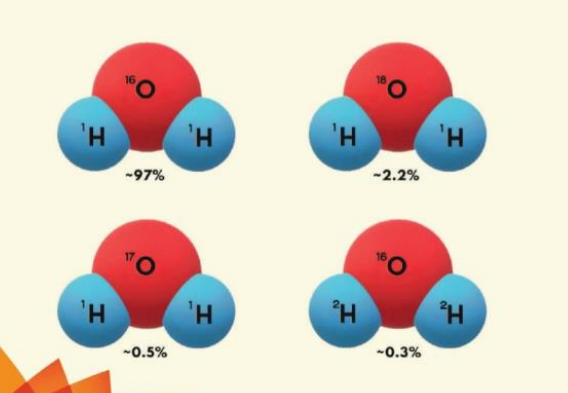
Isotopes are atorms of a particular element having the same atomic number but different atomic mass e.g, there are three isotopes of Oxygen (O, "O and "O) and three isotopes of Hydrogen ('H, "H and "H). Stable isotopes are nuclei that do not decay to other isotopes and Radioactive isotopes are nuclei that spontaneously disintegrate over time to form other isotopes.
Water molecules are made up of hydrogen and oxygen atoms, therefore the isotopes of hydrogen and oxygen are of particular interest in groundwater studies as they act as natural tracers to trace the movement of water. Natural water contains mainly atoms of hydrogen of mass 1 (H) and oxygen of mass 16 ("O). In addition, it also contains small amounts of deuterium (H or D), tritium (H) and isotopes of oxygen like "O and "O. The six isotopes of hydrogen and oxygen may combine to form 18 possible types of water molecule. These different molecules are known as Isotopologues. However, out of 18 possible isotopologues of water, the most common ones are 'H, "O accounting for-97%; 'H, O for- 2.2%; 'Η,ΠΟ for about 0.5% and "H, "O for about 0.3%.
Stable isotopes are not reported as absolute numbers but their isotopic concentrations are expressed as the difference between measured ratio of sample [e.g. (H/H)] to the reference standard [e.g. (H/H) VSMOW where VSMOW is Vienna Standard Mean Ocean Water]. This is expressed as delta (5) notation. Since the value is very small so it is multiplied by 1000 to express in parts per thousand or per mil (%).
The Global Meteoric Water Line (GMWL) describes the global annual average relationship between 8"O and &D in precipitation. It has been found that despite the complexities in various components of hydrological cycle, the &D and 8O in precipitation exhibit a predictable relationship on a global scale which is expressed by the following equation
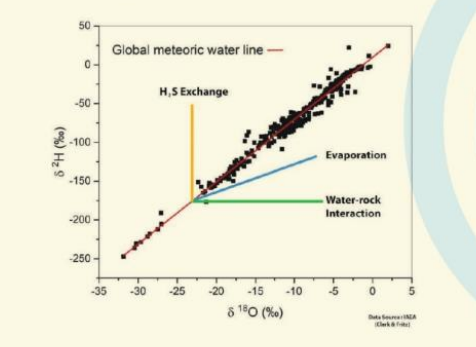
GMWL, thus serves as a reference for interpreting the processes that may lead to modification in the isotopic ratios. In case of groundwater, if the measured values plot away from the line, the type of shifting provides important clues regarding the process leading to the observed shift (e.g. rock-water interaction, evaporation, mixing with seawater, etc.).
Deuterium excess is a derived parameter which is important in interpretation of the isotopic data. The parameter d-excess statics is defined by the relationship d-excess = δ2H − 8 * δ18O (Dansgaard, 1964). This parameter d-excess identifies the relative magnitude of kinetic fractionation in different water and provide information regarding various origins and conditions of formations of the vapour. On a global basis, d-excess averages about 10% but regionally it varies due to variation in humidity, wind speed and sea surface temperature. Higher values of d-excess are found in vapour generated under low humidity conditions.
The dynamics of the water cycle is governed by a series of exchanges among the four major reservoirs of water i.e. the Oceans, the ice caps, fresh water (rivers and lakes) and the atmosphere. The circulation of water on the planet and the various stages of water cycle have been studied in terms of isotopes. The water molecules store the information about their origin and pathways in their isotopic composition. In the hydrological cycle, the ratio of the stable isotopes of H and O changes due to the process of isotopic fractionation which occurs whenever there is change in phase of water i.e during evaporation, condensation, melting and freezing. This causes redistribution of the isotopic molecular species between two phases of the same compound or between two different compounds. During the process of change in phase of water, the heavier isotopes ie the "O and "H or D tend to enrich in the denser phase. Thus, water is enriched in heavier isotopes compared to the corresponding vapour.
Some of the important factors that govern the extent of isotopic fractionation include the evaporation, latitude, altitude and distance from the ocean. Thus, we can get important clues on source of the groundwater through stable isotope composition for e.g., groundwater having source of recharge at higher altitudes will be richer in lighter isotopes and can be distinguished from groundwater recharged at lower altitudes. Similarly, contribution from surface water bodies like reservoirs, ponds and lakes subjected to evaporation can be identified by enrichment in heavier isotopes. Isotopic ratios also help in studying the possible mixing of different types of groundwater, salinisation process and water-rock interaction.
Some of the potential applications of isotopic studies in groundwater investigations include
Dwivedi Yashasvee, Mishra Siddhant, Tiwari Shruti , Verma Ayush, Verma Cherrie , Babu Prashant, Dwivedi S N, Ray R K & Chowdhuri S R (2024), OASIS-G - a webpage for analysis of groundwater stable isotope, data developed under SIH 2022 , Central Ground Water Board (CGWB), DoWR , RD & GR, Ministry of Jal Shakti , Govt. of India.